Deep brain stimulation
List of Deep brain stimulation devices:
Deep brain stimulation (DBS) is a neurosurgical technique in which electrodes are implanted into patient's brain. Although, patients have to undergo an invasive surgery, it is growing field of neurosurgery.[1] DBS proved to be cost-effective in a treatment of certain severe neurological diseases as Parkinson's disease, essential tremor, dystonia, obsessive-compulsive disorder (OCD) or epilepsy.[2] The treatment of other neurological and psychiatric diseases as depression or chronic pain is examined intensively.[1]
DBS is not a cure. None of the diseases to which is used are curable at the moment. It could however, eliminate symptoms of the diseases, which negatively affected the lives of the patients.[3] DBS is used in medical devices, which are on prescription, the use for cognitive enhancement is highly limited. However, certain cases of possible cognitive enhancement were also reported.[4]
Contents
Main Characteristics
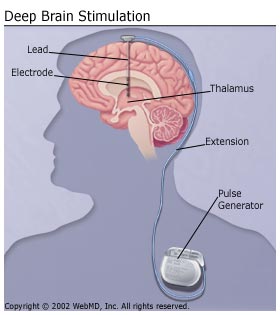
Deep brain stimulation consists in stimulation of a brain by the electrodes, which are implanted in the brain. The electrodes are often referred as leads and are linked with pulse generator via wires. The different diseases could be treated with one device, since the output of the treatment is dependant at the position of electrodes.[5] The pulse generator could be rechargeable or non-rechargeable[6] and could be implanted in the chest[7] or under the scalp[8]. The precise function of the pulse generator is adjusted by the programmer.[5]
The surgery usually consists of two procedures. Firstly, the electrodes are implanted into patient's brain. Patient's head is fixed in a stereotactic head frame, which avoids any movement during the surgery. The patient has to be awake in order to surgeons could see the impact of the stimulation.[5] There are several electronic devices, which could help surgeons to reach the correct spot in the brain.[9] Since DBS does not require a destruction of any area in the brain, it is reversible procedure.[10] A few days later, surgeons could implant the pulse generator.[5] The patients have to visit their doctors several time after the surgery to program correctly their pulse generator. The pulse generators could deliver a various voltage and frequency and usually just a particular programme has the best effect on patient's symptoms.[9]
There are several types of deep brain stimulation. Certain devices as Activa® or Brio™ Rechargeable IPG, delivers open-loop stimulation, which means that the pulses of the current are delivered constantly. This type of devices could be also divided into constant-voltage devices, as are certain devices developed by Medtronic Inc. and constant-current devices, which is for instance Brio™ Rechargeable IPG.[11] In contrast to open-loop systems, closed-loop systems deliver stimulation only in the case the stimulation is needed.[12] The example of the closed-loop system of deep brain stimulation could be RNS® System.[13]
Historical overview
The first reported neurosurgery was conducted in South America approximately 5000 B.C. It consists in boring of holes into the scull of patients as a treatment of neurologic and psychiatric diseases. It was believed that these diseases were caused by the maleficent spirit, who would be eliminated through the hole.[2] The first deep brain stimulation was used for the treatment of human patients in the middle of the 20th century. It was focused on mental and movement disorders. The mortality rate was high, however, and the efficient drug appeared soon after the introduction of DBS. Therefore, the method was abandoned at that time.[14] In addition, FDA required a strict regulation of medical devices since 1976. Therefore, deep brain stimulation system have to proved that they are cost-effective before their used as a treatment.[15]
The renaissance of deep brain stimulation started in late 1980s, when Alim-Louis Benabid use DBS as a treatment for Parkinson's disease and dystonia. His research was accepted better than those of his precursor, even though, there were no considerable difference in his approach. The important factor was that in the period, when he is conducted his research, the neurologists were looking for an alternative to levodopa, the drug which was used as a treatment of Parkinson's disease. They faced the fact that the drug lost its effectiveness in the long run. In addition Benabid successfully identified regions in the brain which should be stimulated.[15] DBS had been provided at humanitarian device exemption at first, but it was approved as a treatment of Parkinson's disease and essential tremor in 1997.[16] It has also pre-market approval for a treatment of dystonia,[17] epilepsy[18] and OCD[2].
Purpose
Deep brain stimulation was developed as a treatment of neurological and psychiatric diseases, in the cases when medication is unsuccessful.
Important Dates
- about 5000 B.C. the first trephinations were conducted in South America.[2]
- 1987 - the research on deep brain stimulation of the thalamic nucleus ventralis intermedius to treat symptoms of Parkinson's disease was published[19]
- 1993 - deep brain stimulation of subthalamic nucleus was introduced as a treatment of Parkinson's disease.[20]
- 1997 - deep brain stimulation was approved as a treatment of essential tremor by FDA.[7]
- 2002 - deep brain stimulation was approved as a treatment of Parkinson's disease by FDA.[15]
Enhancement/Therapy/Treatment
Enhancement
Although DBS is primarily used in medicine, it has also certain potential in cognitive enhancement. Clement Hamani and his colleagues reported that during the surgery and DBS treatment of one of their patients, certain cognitive enhancement appeared. The patient suffered from morbid obesity and since the other attempts to reduce his weight fell or were refused, they suggested DBS. In order to affect obesity, they decided to stimulate hypothalamus, which is also linked with memory. After the stimulation of hypothalamus, patient reported an enhancement of memory. The enhancement was also proved in testing, namely the patient scored significantly better in California Verbal Learning Test and Spatial Associative Learning Test.[4]
Parkinson's disease
Parkinson's disease is the neurodegenerative disease, which is caused by a loss of neurons in the substantia nigra pars compacta. These neurons produce dopamine, therefore patients suffer from the loss of dopamine. It causes tremor, postural instability and bradykinesia. Patients could be treated by levodopa or other dopaminergic drugs but these drugs have side effects in the long-term use and tend to loss their efficiency.[21] The accuracy of diagnosis of Parkinson's disease is low, thus deep brain stimulation is appropriate only for those patients, which suffer from it for more than 5 years, in order to assure that they really suffers from Parkinson's disease. Therefore, deep brain stimulation is mainly used among patients, whose medication cannot sufficiently handle with the symptoms of Parkinson's disease and the diagnosis of the disease was confirmed.[7] However, each candidate for DBS as a treatment of Parkonson's disease has to be reviewed carefully by the doctor, in order to decide that the treatment is appropriate and cost-efficient for him or her.[10]
As a treatment of Parkinson's disease is usually used the stimulation of one of these three areas of brain: the thalamus, globus pallidus pars interna (GPi), or subthalamic nucleus (SNT), but the stimulation of SNT is preferred at present.[21] The surgeons choose also the location of electrodes with respect to the symptoms of Parkinson's disease, which they intend to affect. Fukuya and Yamamoto argue that certain Parkinson's patients would benefit from DBS of GPi, while other symptoms are better reduced by the stimulation of SNT.[22] Breit and his colleagues claim that recently DBS turned to be the most frequently used surgical therapy for movement disorders.[10]
Essential tremor
Essential tremor is a movement disorder, which appears mainly among population over 60. It consists in oscillating movements of parts of patient's body, particularly upper limbs. The cause of this disorder is unknown and it is not curable at present. The treatment is based on the elimination of its symptoms. The surgical therapy of essential tremor is focused on patients, whom medication did not provide relief. Besides deep brain stimulation, patients could undergo also lesional therapies, which are however more invasive than DBS.[23]
The stimulation of the ventro-intermedius nucleu (VIM) is prevalently used as a treatment of essential tremor.[24]
Dystonia
Dystonia is a movement disorder, which consists in muscle contractions. The contractions usually cause twisting, repetitive movements or abnormal postures. Dystonia could be divided into primary and secondary dystonia. Primary dystonia might be inherited and it is not linked with other neurological disorder. Secondary dystonia, in contrast, could be caused by brain injury, intoxication, or drug's abuse. Secondary dystonia is further linked with other neurological sighs.[25]
Two types of stimulation have been used for a treatment of dystonia, pallidal stimulation (stimulation of GPi) and thalamic stimulation. While benefits of pallidal stimulation were documented, there are not sufficient data to claim benefit of thalamic stimulation.[26] The stimulation of GPi have positive impact mainly on primarily dystonia, in the case of secondary dystonia the responses considerably vary.[16]
Epilepsy
Epilepsy is a common neurological disorder which affects approximately 50 million people worldwide.[27] Epilepsy is caused by the disturbance of normal pattern of brain activity. This disturbance manifests as the seizure usually accompanied with convulsions, muscle spasms, and loss of consciousness. The seizures are affected by various factors as illness or abnormal brain development.[28] Considerably great number of patients suffering from epilepsy (approximately 30%) cannot control their condition by medication. Deep brain stimulation therapy is one of the possibilities for these patients to handle their conditions.[27]
There are several neurostimulation therapies focused on the treatment of epilepsy. Besides Vagus Nerve Stimulation and Trigeminal Nerve Stimulation, there are various therapies of deep brain stimulation which differ in the placement of electrodes and in the way they delivers the current. From the point of view of the current, there are open-loop systems and closed-loop systems of DBS therapy for epilepsy. The former therapy consists in the constant delivery of the current in the certain area of patient's brain. The areas which were stimulated in epilepsy studies are anterior nucleus of the thalamus (ANT), cerebellum, centromedian nucleus of the thalamus, hippocampus, caudate nucleus, subthalamic nucleus, posterior hypothalamic mammilary nuclei, and locus ceruleus. The closed-loop therapy deliver the current only if the system reports the seizure, therefore the system have to monitor the brain activity constantly. In this therapy, electrodes are placed in the epileptogenic foci. At most two distinct epileptogenic foci could be stimulated, therefore the method is not suitable for patients with more distinct epileptogenic foci. In addition, patient's epileptogenic foci have to be known to the surgeon.[29]
Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD) is a neurological disorder, which pushes patients to a repetitive behaviour or mental acts. The behaviour serves as the neutralisation of anxiety produced by obsessions, intrusive and senseless fears and images, which occupy patients' minds. OCD impairs patient's goal-directed behaviour, socio-economic status and social interaction in general. Patients could be treated by medications or behavioural therapy, but approximately 10% of all patients do not respond to these types of therapies. DBS was suggested as the treatment for this OCD patients recently.[30]
The most beneficial DBS therapy for the treatment of OCD is still under review. There were suggested several areas in the brain for DBS stimulation of OCD patients, but the most used are the stimulation of the rostral–caudal extent of the anterior limb of the internal capsule and/or the adjacent striatum,[31] nucleus accumbens, and subthalamic nucleus.[30]
DBS is at present used as a treatment of wide range of diseases in research, but its use has not been approved by FDA yet. They are diseases as anorexia nervosa,[32] Alzheimer’s disease,[33] treatment-resistant depression,[34] neuropathic pain[35] obesity,[4] or Tourette syndrome.[36]
Ethical & Health Issues
DBS could considerably improved patient's life, but it is not without risks. There are several health and ethical issues, which could negatively affected patient's life, which were reported.[30][37]
The health issues could be divided into three type. Firstly, there are health issues which are related to surgery. Although DBS is considered to be non-invasive procedure, since it is reversible, there is still danger that the surgery will be life threatening. The most serious complications linked with deep brain stimulation are haemorrhage and infection. These complications might cause the dead of patient. The surgery could also lead to headaches, scalp tingling or numbness or convulsions.[30] Secondly, further issues are linked with the device. The therapy could be negatively affected by the migration of electrodes, the malfunction of a neurostimulator, breaking of the electrodes or wires.[38] Moreover, the device could also negatively interfere with devices that radiate as microwave, mobile phones, appliances containing magnet, theft detectors, metal screening devices, or other implanted devices as peacemaker. The patients with neuromodulators and implanted electrodes should also avoid certain therapies and treatments as electroshock therapy, transcranial magnetic stimulation, diathermy, magnetic resonance, external defibrillators, and therapeutic radiation.[39] Finally, certain issues could be caused by stimulation. Patients which underwent DBS struggle with several issues as confusion, dysarthria, fatigue, oedema,[40] dizziness, or anxiety.[30]
In addition, there are issues linked with personal and social life of patients. Although the social life improve in general, patients reported the enlarge rate of the divorce after DBS. It might be caused by the change of caregiver-patient's dynamic in the married couples, in which one of them received DBS. Moreover, the suicidal wishes and attempts are increased by DBS. DBS also stimulate impulsive behaviour as hypersexuality, gambling or addiction on video games.[37]
Public & Media Impact and Presentation
There are several stories on Youtube, where people or journalists describe DBS therapy as a treatment of neurological and psychiatric diseases Parkinson's disease,[41][42] dystonia[43][44] depression,[45][46] and OCD.[47] In general, patients reported positive experience with DBS on Youtube. The claims:
- "This actually change my life very much. It's not only giving me back my dignity [...] It enables me to do some fantastic things."[42]
- "It is the greatest decision I ever made in my entire life."[44]
- "I didn't even know that life could be this much 'cause I've just always been depressed."[45]
- "The surgery has made a huge difference in my life. No more shaking, no more dystonia, and very little dyskinesia. I can get dressed by myself, shower, I can type and write again, and eventually walk normally again. I have no regrets at all. I am very happy and I just want to say thank you to Dr. Rezak for everything. You are the best!"[48]
In contrast, Ian Frizell focused in his entry in the side-effects that are not sufficiently stressed before DBS surgery. He listed responses from his survey, which he conducted among patients who underwent DBS surgery. Among these side-effects he listed dyskinesia, the recovery of patient's appetite, impulsive behaviour, disturbance of vision, light-headedness or dizziness, back pain, cognitive impairment, and the cessation of constipation. Frizell points out that certain side-effects could be removed or mitigated by the programming of the neuromodulator. He also reported that one person had an allergic reaction, which is, however, an complication not the side-effect according to Frizell. He concluded that besides one patient, who had severe surgery complications, all patients would underwent DBS surgery again, since it changed their life in a positive way.[41] There is also web page which collects the experience of patients, who underwent DBS.[49]
Some posts are focused on encouraging patient's which could benefit from DBS:
This blog is created in the hopes that my experiences will give others inspiration to take a risk and have the courage to face the unknown in order to accomplish the impossible as others have done for me.[50]
There are posts, which warns patients, who considers DBS, that it is not a miracle cure as:
If you are expecting a quick fix, you will be disappointed. First your brain has to heal from the surgery (8 - 12 weeks), then the settings must be adjusted SLOWLY. Finding the right combination of settings and medications takes time. In my own case, it took OVER A YEAR to get my settings optimized. Notice I said optimized, not perfect. My settings and how much medication I take has been adjusted several times in years number two and three. I can't tell you the number of people who contact me and complain that their symptoms aren't gone when their generator has just been turned on. It takes time.[51]
Some people with Parkinson’s Disease (PD) who have had Deep Brain Stimulation (DBS) surgery say that their lives have turned out for the better through the miracle of DBS. Others are disappointed when the results of DBS don’t meet their expectations. Successful DBS is related to realistic expectations.[52]
Davis Phinney the former American cyclist was diagnosed by Parkinson's disease when he was 40. He was treated with DBS and reported that it brought him relief:
"The doctor said, 'OK, let's try a little current now," Davis remembers, "and just like that, all these muscles that had been at war with each other suddenly were at peace. It was like Armistice Day. It was just like, "Oh ... my ... god! I looked at my wife and she was crying. She said, 'I haven't seen your smile in a year!'"[53]
However, his tremor came back in four years.[53] Maria de Leon argues that there is a discrepancy in the prescriptions of DBS:
However, the worst part in my opinion is the huge discrepancy seen in those implanted, at least here in this country, which tend to be white males (95%). Even when women and other minorities are seeing and being followed by the same specialist as the men implanted, these groups seldom even get the option of DBS as a potential treatment.[54]
Edi Guyton reported that at an airport they were afraid of her when she admitted that she did not have pacemaker but electrodes in her brain. They thought she would explode.[46]
Public Policy
Medtronic DBS Therapy for Parkinson's disease was approved by (U.S. Food and Drug Administration) FDA in 2002.[55]
Brio™ Rechargeable IPG was approved as a treatment of Parkison's disease and essential tremor by FDA in 2015.[56]
DBS is approved in Europe, Canada and Australia as a treatment of refractory epilepsy. It is also approved as a treatment of epilepsy[18] and the treatment-resistant OCD under Humanitarian Device Exemption in the United States.[55]
Brio™ Rechargeable IPG received CE (Conformité Européenne) mark of approval for the treatment of dystonia.[57]
Related Technologies, Projects or Scientific Research
There are various alternative therapies which are used as a treatment of diseases which are also treated by deep brain stimulation. In many cases patients receive medication, even though there is only an off-label treatment for essential tremor[12] and medications for Parkinson's disease tend to wear off.[21] There is also behavioural therapy available for psychiatric disorders as OCD.[30] Nonetheless, there are patients whom these therapies do not bring relief. Other therapies used for the treatment of the diseases and disorders treated with DBS are lesional therapies, transcranial magnetic stimulation,[12] transcranial direct-current stimulation[58] vagus nerve stimulation,[59] trigeminal nerve stimulation.[60]
References
- ↑ 1.0 1.1 GOSSET, Nathalie, DIETZ, Nicholas. IEEE Pulse [online]. Unlocking Pain: Deep brain stimulation might be the key to easing depression and chronic pain. 2015, Mar 15. Available online at: http://pulse.embs.org/march-2015/unlocking-pain/ (Retrieved 13th April, 2017).
- ↑ 2.0 2.1 2.2 2.3 LOZANO, Andres. Tuning the Brain. The Scientist [online]. 2013, Oct 28. Available online at: http://www.the-scientist.com/?articles.view/articleNo/38047/title/Tuning-the-Brain/ (Retrieved 12th April, 2017).
- ↑ U.S. Food and Drug Administration. FDA approves brain implant to help reduce Parkinson’s disease and essential tremor symptoms. U.S. Food and Drug Administration [online]. 2015, Jun 12. Available online at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm451152.htm (Retrieved 6th April, 2017).
- ↑ 4.0 4.1 4.2 HAMANI, Clement. et al. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Annals of Neurology, 2008, 63(1), 119–123. Doi: 10.1002/ana.21295 Available online at: http://ve5kj6kj8s.scholar.serialssolutions.com/?sid=google&auinit=C&aulast=Hamani&atitle=Memory+enhancement+induced+by+hypothalamic/fornix+deep+brain+stimulation&id=doi:10.1002/ana.21295&title=Annals+of+neurology&volume=63&issue=1&date=2008&spage=119&issn=0364-5134 (Retrieved 13th April, 2017).
- ↑ 5.0 5.1 5.2 5.3 NORDQVIST, Joseph. What is Deep Brain Stimulation?. MedicalNewsToday [online]. 2016, Sep 7. Available online at: http://www.medicalnewstoday.com/articles/265445.php?utm_source=TrendMD&utm_medium=cpc&utm_campaign=Medical_News_Today_TrendMD_0 (Retrieved 11th April, 2017).
- ↑ GILLIES, Martin J. et al. Rechargeable vs. Nonrechargeable Internal Pulse Generators in the Management of Dystonia. Neuromodulation: Technology at the Neural Interface, 2013, 16(3), 226-229. Doi: 10.1111/ner.12026 Available online at: http://onlinelibrary.wiley.com/doi/10.1111/ner.12026/full (Retrieved 11th April, 2017).
- ↑ 7.0 7.1 7.2 DANCE, Amber. Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD. Alzforum [online]. 2010, May 21. Available online at: http://www.alzforum.org/news/research-news/deep-brain-stimulation-theres-still-room-improvement (Retrieved 12th April, 2017).
- ↑ SIRVEN, J.I. Responsive Neurostimulation. Epilepsy Foundation [online]. 2014, May. Available online at: http://www.epilepsy.com/learn/treating-seizures-and-epilepsy/devices/responsive-neurostimulation (Retrieved 13th April, 2017).
- ↑ 9.0 9.1 DANCE, Amber. Deep-Brain Stimulation: There’s Still Room for Improvement. Alzforum [online]. 2010, May 25. Available online at: http://www.alzforum.org/news/research-news/deep-brain-stimulation-electrode-all-occasions (Retrieved 13th April, 2017).
- ↑ 10.0 10.1 10.2 BREIT, Sorin et al. Deep Brain Stimulation. Cell and Tissue Research, 2004, 318(1), 275–288. Doi: 10.1007/s00441-004-0936-0 Available online at: http://link.springer.com/article/10.1007/s00441-004-0936-0 (Retrieved 13th April, 2017).
- ↑ PREDA, F. et al. Switching from constant voltage to constant current in deep brain stimulation: a multicenter experience of mixed implants for movement disorders. European Journal of Neurology, 2016, 23(1), 190–195. Doi:10.1111/ene.12835 Available online at: http://onlinelibrary.wiley.com/doi/10.1111/ene.12835/full (Retrieved 6th April, 2017).
- ↑ 12.0 12.1 12.2 PICILLO, Marina, FASANO, Alfonso. Recent advances in Essential Tremor: Surgical treatment. Parkinsonism & Related Disorders, 2016, 22(1), S171–S175. DOI: 10.1016/j.parkreldis.2015.09.012 Available online at: http://www.sciencedirect.com/science/article/pii/S1353802015003879 (Retrieved 6th April, 2017).
- ↑ WU, C., SHARAN, A.D. Neurostimulation for the Treatment of Epilepsy: A Review of Current Surgical Interventions. Neuromodulation, 2013, 16, 10–24. Doi: 10.1111/j.1525-1403.2012.00501.x Available online at: http://onlinelibrary.wiley.com/doi/10.1111/j.1525-1403.2012.00501.x/abstract (Retrieved 13th April, 2017).
- ↑ SCHWALB, Jason M., HAMANI Clement. The history and future of deep brain stimulation. Neurotherapeutics. 2008, Jan. 5(1), 3–13. Doi: 10.1016/j.nurt.2007.11.003 Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5084122/ (Retrieved 13th April, 2017).
- ↑ 15.0 15.1 15.2 GARDNER, John. A history of deep brain stimulation: Technological innovation and the role of clinical assessment tools. Social Studies of Science. 2013, Oct. 43(5), 707–728. Doi: 10.1177/0306312713483678 Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3785222/#bibr79-0306312713483678 (Retrieved 13th April, 2017).
- ↑ 16.0 16.1 MIOCINOVIC, Svjetlana et al. History, Applications, and Mechanisms of Deep Brain Stimulation. Neurological Review. 2013, 70(2), 163-171. Doi: 10.1001/2013.jamaneurol.45 Available online at: http://jamanetwork.com/journals/jamaneurology/fullarticle/1391074 (Retrieved 19th April, 2017).
- ↑ University of Wisconsin Hospitals and Clinics. Neurosurgery: Deep Brain Stimulation (DBS) Frequently Asked Questions. University of Wisconsin Hospitals and Clinics [online]. 2017. Available online at: http://www.uwhealth.org/neurosurgery/deep-brain-stimulation-dbs-frequently-asked-questions/12764 (Retrieved 19th April, 2017).
- ↑ 18.0 18.1 Business Wire. FDA Grants Premarket Approval (PMA) for the NeuroPace® RNS® System to Treat Medically Refractory Epilepsy. Business Wire [online]. 2013, Nov 14. Available online at: http://www.businesswire.com/news/home/20131114006420/en/3073855/FDA-Grants-Premarket-Approval-PMA-NeuroPace%C2%AE-RNS%C2%AE (Retrieved 19th April, 2017).
- ↑ BENABID A.L. et al. Combined (Thalamotomy and Stimulation) Stereotactic Surgery of the VIM Thalamic Nucleus for Bilateral Parkinson Disease. Appl Neurophysiol, 1987, 50, 344–346 Doi: 10.1159/000100803 Available online at: https://www.karger.com/Article/Abstract/100803 (Retrieved 12th April, 2017).
- ↑ BENABID A.L. et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg, 1994, 62, 76–84. Doi: 10.1159/000098600 Available online at: https://www.karger.com/Article/Abstract/98600 (Retrieved 12th April, 2017).
- ↑ 21.0 21.1 21.2 WILLIAMS, Adrian et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurology. 2010, 9(6), 581–91. Doi: 10.1016/S1474-4422(10)70093-4 Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2874872/ (Retrieved 19th April, 2017).
- ↑ FUKAYA, Chikashi and YAMAMOTO, Takamitsu. Deep Brain Stimulation for Parkinson’s Disease: Recent Trends and Future Direction. Neurologia medico-chirurgica. 2015, 55(5), 422–431. Doi: 10.2176/nmc.ra.2014-0446 Available online at: https://www.ncbi.nlm.nih.gov/pubmed/25925761 (Retrieved 21st April, 2017).
- ↑ PICILLO, Marina, FASANO, Alfonso. Recent advances in Essential Tremor: Surgical treatment. Parkinsonism & Related Disorders, 2016, 22(1), S171–S175. DOI: 10.1016/j.parkreldis.2015.09.012 Available online at: http://www.sciencedirect.com/science/article/pii/S1353802015003879 (Retrieved 6th April, 2017).
- ↑ BAIZABAL-CARVALLO, J. F. et al. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. Journal of Neurology, Neurosurgery & Psychiatry, 2014, 85(5), 567-72. Doi: 10.1136/jnnp-2013-304943. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/24096713 (Retrieved 21st April, 2017).
- ↑ ANDREWS, Caroline et al. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. Journal of Neurology, Neurosurgery and Psychiatry, 2010, 81(12), 1383-1389. Doi: 10.1136/jnnp.2010.207993 Available online at: http://jnnp.bmj.com/content/81/12/1383.long (Retrieved 19th April, 2017).
- ↑ TAGLIATI, Michele et al. Long-Term management of DBS in dystonia: Response to stimulation, adverse events, battery changes, and special considerations. Movement disorders. 2011, 26(1), 54-62. Doi: 10.1002/mds.23535 Available online at: http://onlinelibrary.wiley.com/doi/10.1002/mds.23535/abstract (Retrieved 19th April, 2017).
- ↑ 27.0 27.1 KLINGER, Neil V., MITTAL, Sandeep. Clinical efficacy of deep brain stimulation for the treatment of medically refractory epilepsy. Clinical Neurology and Neurosurgery, 2016, 140, 11–25. Doi: 10.1016/j.clineuro.2015.11.009 Available online at: http://www.sciencedirect.com/science/article/pii/S0303846715300755 (Retrieved 21st April, 2017).
- ↑ National Institute of Neurological Disorders and Stroke. Epilepsy Information Page. National Institute of Neurological Disorders and Stroke [online]. Available online at: https://www.ninds.nih.gov/Disorders/All-Disorders/Epilepsy-Information-Page (Retrieved 21st April, 2017).
- ↑ WU, C., SHARAN, A.D. Neurostimulation for the Treatment of Epilepsy: A Review of Current Surgical Interventions. Neuromodulation, 2013, 16, 10–24. Doi: 10.1111/j.1525-1403.2012.00501.x Available online at: http://onlinelibrary.wiley.com/doi/10.1111/j.1525-1403.2012.00501.x/abstract (Retrieved 13th April, 2017).
- ↑ 30.0 30.1 30.2 30.3 30.4 30.5 ALONSO, Pino et al. Deep Brain Stimulation for Obsessive-Compulsive Disorder: A Meta-Analysis of Treatment Outcome and Predictors of Response. PLoS One. 2015, 10(7), e0133591. Doi: 10.1371/journal.pone.0133591. Available online at: https://www.ncbi.nlm.nih.gov/pubmed/26208305 (Retrieved 24th April, 2017).
- ↑ GREENBERG, B. D. et al. Greenberg Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Molecular Psychiatry 2010, 15(1), 64–79. Doi: 10.1038/mp.2008.55 Available online at: https://www.ncbi.nlm.nih.gov/pubmed/26208305 (Retrieved 24th April, 2017).
- ↑ LIPSMAN, Nir et al. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. The Lancet: Psychiatry. 2017, 4(4), 285–294. Doi: 10.1016/S2215-0366(17)30076-7 Available online at: http://www.thelancet.com/journals/lanpsy/article/PIIS2215-0366(17)30076-7/fulltext (Retrieved 24th April, 2017).
- ↑ NARDONE, Raffaele et al. Neurostimulation in Alzheimer’s disease: from basic research to clinical applications. Neurological Sciences. 2015, 36(5), 689–700. Available online at: http://link.springer.com/article/10.1007/s10072-015-2120-6 (Retrieved 24th April, 2017).
- ↑ MAYBERG, Helen S. et al. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005, 45(5), 651–660. Available online at: http://www.sciencedirect.com/science/article/pii/S089662730500156X (Retrieved 24th April, 2017).
- ↑ GRAY, Alan M. et al. Deep Brain Stimulation as a Treatment for Neuropathic Pain: A Longitudinal Study Addressing Neuropsychological Outcomes. The Journal of Pain, 2014, 15(3), 283–292. Doi: 10.1016/j.jpain.2013.11.003 Available online at: http://www.sciencedirect.com/science/article/pii/S1526590013013849 (Retrieved 24th April, 2017).
- ↑ SERVELLO, D. et al. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. Journal of Neurology, Neurosurgery & Psychiatry, 2008, 79, 136-142. Doi: 10.1136/jnnp.2006.104067 Available online at: http://jnnp.bmj.com/content/79/2/136.short (Retrieved 24th April, 2017).
- ↑ 37.0 37.1 DANCE, Amber. Deep-Brain Stimulation: Steadies the Body, But What About the Mind?. Alzforum [online]. 2010, May 24. Available online at: http://www.alzforum.org/news/research-news/deep-brain-stimulation-steadies-body-what-about-mind (Retrieved 24th April, 2017).
- ↑ U.S. Food and Drug Administration [online]. Summary of Safety and Effectiveness Data. U.S. Food and Drug Administration [online]. Available online at: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140009b.pdf (Retrieved 24th April, 2017).
- ↑ St. Jude Medical. Brio™ Patient Programmer. U.S. Food and Drug Administration [online]. Available online at: www.accessdata.fda.gov/cdrh_docs/pdf14/P140009c.pdf (Retrieved 6th April, 2017).
- ↑ OKUN, M. et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. The Lancet: Neurology, 2012, 11(2), 140-149. Doi: 10.1016/S1474-4422(11)70308-8 Available online at: http://www.sciencedirect.com/science/article/pii/S1474442211703088 (Retrieved 6th April, 2017).
- ↑ 41.0 41.1 FRIZELL, Ian. Deep Brain Stimulation (DBS) side effects. Youtube [online]. 2016, Oct 27. Available online at: https://www.youtube.com/watch?v=7wYfafhuITA (Retrieved 25th April, 2017).
- ↑ 42.0 42.1 WIRED Events. How a Brain Implant Gave a Parkinson's Patient Back His Life: WIRED Health 2017: WIRED Events. Youtube [online]. 2017, Mar 23. Available online at: https://www.youtube.com/watch?v=fDz6R82h6qU (Retrieved 25th April, 2017).
- ↑ Children's Mercy. Deep Brain Stimulation at Children's Mercy. Youtube [online]. 2016, Aug 8. Available online at: https://www.youtube.com/watch?v=jXhfGA56v9Q (Retrieved 25th April, 2017).
- ↑ 44.0 44.1 BionicDystonic. Deep Brain Stimulation: A Patient's Perspective. Youtube [online]. 2016, Jul 7. Available online at: https://www.youtube.com/watch?v=aHxd_IIbxE4 (Retrieved 25th April, 2017).
- ↑ 45.0 45.1 Department of Clinical Neurosciences. Deep Brain Stimulation (DBS) for treatment-resistant depression. Youtube [online]. 2016, May 18. Available online at: https://www.youtube.com/watch?v=L_c2yI5otTM (Retrieved 25th April, 2017).
- ↑ 46.0 46.1 Stephan Hamann. Deep Brain Stimulation for Depression CNN Presents Special with Dr. Sanjay Gupta. Youtube [online]. 2012, May 6. Available online at: https://www.youtube.com/watch?v=Lq5rIILcVgA (Retrieved 26th April, 2017).
- ↑ CNN. Sanjay Gupta MD: Deep brain stimulation helps OCD patient. Youtube [online]. 2014, Jun 23. Available online at: https://www.youtube.com/watch?v=LON36XhpdDs (Retrieved 26th April, 2017).
- ↑ MILKE, Steven. Unique Aspects of the Diagnosis & Treatment of YOPD: A Patient’s Perspective. American Parkinson Disease Association [online]. 2010, Feb 13. Available online at: https://www.apdaparkinson.org/story/steve/ (Retrieved 26th April, 2017).
- ↑ PatientsLikeMe. Deep Brain Stimulation treatment report. PatientsLikeMe [online]. Available online at: https://www.patientslikeme.com/ms/patients/treatment/991#overview (Retrieved 26th April, 2017).
- ↑ Focus on a Cure Foundation. Headline. Focus on a Cure Foundation [online]. Available online at: http://focusonacure.blogspot.cz/ (Retrieved 26th April, 2017).
- ↑ YumaBev. Welcome to My Story about DBS. My DBS Story [online]. Available online at: http://katekelsall.typepad.com/my_weblog/parkinsons_deep_brain_stimulation/ (Retrieved 26th April, 2017).
- ↑ KELSALL, Kate. Expectations and Acceptance of DBS. Shake, Rattle and Roll: An insider's view of Parkinson's Disease and DBS [online]. 2016, Jun 26. Available online at: http://katekelsall.typepad.com/my_weblog/parkinsons_deep_brain_stimulation/ (Retrieved 26th April, 2017).
- ↑ 53.0 53.1 REILLEY, Rick. Riding it out. ESPN [online]. 2012, Aug 21. Available online at: http://www.espn.com/espn/story/_/id/8194683/davis-taylor-phinney (Retrieved 25th April, 2017).
- ↑ DE LEON, Marie. Taking a Closer Look at DBS: By Dr. De Leon. defeatPARKINSONS [online]. 2015, Nov 6. Available online at: https://defeatparkinsons.com/2015/11/06/taking-a-closer-look-at-dbs-by-dr-de-leon/ (Retrieved 26th April, 2017).
- ↑ 55.0 55.1 Medtronic plc. FDA Approves Medtronic Deep Brain Stimulation for People with Parkinson's Disease with Recent Onset of Motor Complications. Medtronic plc [online]. Available online at: http://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=2139957 (Retrieved 26th April, 2017).
- ↑ U.S. Food and Drug Administration. FDA approves brain implant to help reduce Parkinson’s disease and essential tremor symptoms. U.S. Food and Drug Administration [online]. 2015, Jun 12. Available online at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm451152.html (Retrieved 26th April, 2017).
- ↑ GUILLIOTTI, Kimberly. St Jude Medical’s Deep Brain Stimulation Trifecta: New Treatment Options for Dystonia in EU. MD Buyline [online]. 2013, Apr 23. Available online at: http://www.mdbuyline.com/st-jude-medicals-deep-brain-stimulation-trifecta-new-treatment-options-for-dystonia-in-eu/ (Retrieved 6th April, 2017).
- ↑ LEFAUCHEUR J.P., ANTAL A., et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 2017, 128(1), 56-92. Doi: 10.1016/j.clinph.2016.10.087 Available online at: http://www.sciencedirect.com/science/article/pii/S1388245716306344 (Retrieved 26th April, 2017).
- ↑ SCHACHTER, S.C., SIRVEN, J.I. Vagus Nerve Stimulation (VNS). Epilepsy foundation [online]. 2013. Available online at: http://www.epilepsy.com/learn/treating-seizures-and-epilepsy/devices/vagus-nerve-stimulation-vns (Retrieved 26th April, 2017).
- ↑ PACK, A.M. Trigeminal Nerve Stimulation May Not Be Effective for the Treatment of Refractory Partial Seizures. Epilepsy Currents. 2013, 13(4), 164–165.